30 October, 2011
Study Points to Potential Treatment for Sickle Cell Disease
Scientists corrected sickle cell disease in adult laboratory mice by activating production of a special blood protein normally produced only before birth. The approach may lead to new treatments for people with the blood disorder.

Sickle cell disease is caused by an abnormality in hemoglobin, the protein in red blood cells that carries oxygen throughout the body. About 100,000 Americans live with sickle cell disease. It is most prevalent in people of African, Hispanic, Mediterranean and Middle Eastern descent.
People with sickle cell disease have 2 copies of an altered hemoglobin gene. The defective protein that results changes shape after releasing its oxygen. This causes red blood cells to become stiff, misshapen and sticky. These sickle-shaped cells slow blood flow to tissues, resulting in organ damage.
There is no widely available cure for sickle cell disease. Bone marrow transplants have cured some patients. However, the treatment poses several risks, and most patients don’t have relatives who can donate compatible, healthy bone marrow.
Past studies have shown that an elevated level of a form of hemoglobin called fetal hemoglobin reduces the tendency of sickle hemoglobin to change the shape of red blood cells. Production of fetal hemoglobin normally predominates before birth but turns off as adult hemoglobin takes over. A drug called hydroxyurea can boost production of fetal hemoglobin and reduce the complications of sickle cell disease. However, not all patients respond well to this medication, and adverse side effects are a concern.
A research team led by Dr. Stuart Orkin set out to explore a more targeted approach to raising fetal hemoglobin by blocking production of a protein called BCL11A. The team—at Harvard Medical School, Children’s Hospital of Boston and the Howard Hughes Medical Institute, Boston—had previously demonstrated that BCL11A suppresses the production of fetal hemoglobin soon after birth. Their work was funded by NIH’s National Heart, Lung and Blood Institute (NHLBI), National Cancer Institute (NCI) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The study appeared in the October 13, 2011, online edition of Science.
The scientists used a genetic technique to inactivate the gene for BCL11A in mice with sickle cell disease. Without BCL11A, the mice continued to produce fetal hemoglobin. Sickled cells were absent in the mice, as were their disease symptoms. Other aspects of blood production appeared to be unaffected.
“This study provides the first proof of principle that BCL11A might serve as a target for treating sickle cell disease and related blood disorders such as the thalassemias,” Orkin says. More research is needed, however, before such therapies can be tested in people.
23 October, 2011
Commonly used three-drug regimen for idiopathic pulmonary fibrosis found harmful
NIH stops one treatment arm of trial; other two treatments to continue
The National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health, has stopped one arm of a three arm multi-center, clinical trial studying treatments for the lung-scarring disease idiopathic pulmonary fibrosis (IPF) for safety concerns. The trial found that people with IPF receiving a currently used triple-drug therapy consisting of prednisone, azathioprine, and N-acetylcysteine (NAC) had worse outcomes than those who received placebos or inactive substances.
"These findings underscore why treatments must be evaluated in a rigorous manner," said Susan B. Shurin, M.D., acting director of the NHLBI. "This combination therapy is widely used in patients with IPF, but has not previously been studied in direct comparison to a placebo for all three drugs."
The interim results from this study showed that compared to placebo, those assigned to triple therapy had greater mortality (11 percent versus 1 percent), more hospitalizations (29 percent versus 8 percent), and more serious adverse events (31 percent versus 9 percent) and also had no difference in lung function test changes. Participants randomly assigned to the triple- therapy arm also remained on their assigned treatment at a much lower rate (78 percent adherence versus 98 percent adherence).
"Anyone on some combination of these medications with questions or concerns should consult with their health care provider and not simply stop taking the drugs," said Ganesh Raghu, M.D., professor of medicine at the University of Washington, Seattle and a co-chair of this IPF study. "It is important to realize that these results definitively apply only to patients with well-defined IPF and not to people taking a combination of these drugs for other lung diseases or conditions."
The other two study arms, or intervention groups, of this IPF trial comparing NAC alone to placebo alone will continue. In stopping this part of the trial, the NHLBI accepted the recommendation of the Data and Safety Monitoring Board (DSMB) – an independent advisory group of experts in lung disease, biostatistics, medical ethics, and clinical trial design. The DSMB has been monitoring the study since it began.
This study, called PANTHER-IPF (Prednisone, Azathioprine, and N-acetylcysteine: A Study that Evaluates Response in Idiopathic Pulmonary Fibrosis) was designed and conducted by the Idiopathic Pulmonary Fibrosis Clinical Research Network, funded by the NHLBI. The PANTHER-IPF study was designed to evaluate whether this commonly used triple-therapy regimen could slow disease progression and improve lung function in people with moderate IPF.
PANTHER-IPF was the first study in IPF comparing the effectiveness of this combined treatment to a placebo for all three drugs. Each participant had a one in three chance of being randomized to receive the triple drug regimen, NAC alone, or placebo for a period of up to 60 weeks.
"We will continue to analyze the data to try to understand why this particular combination may be detrimental in people with IPF," said Fernando Martinez, M.D., professor of medicine, University of Michigan, Ann Arbor and co-chair of the PANTHER-IPF study. "The results are not explained by any differences between the two groups before the treatments started."
IPF is a progressive and currently incurable disease characterized by the buildup of fibrous scar tissue within the lungs. This accumulation of scar tissue leads to breathing difficulties, coughing, chest pain, and fatigue. Approximately 200,000 people in the United States have IPF. The cause or causes of IPF remain unknown; as a result treatment options remain limited. PANTHER-IPF began enrollment in October 2009.
The study had enrolled 238 of a planned 390 participants prior to the stop announcement. Participants ranged from 48 to 85 years of age, with an average age of 68. The placebo and NAC arms will continue enrolling and following their participants, and this part of the PANTHER-IPF study is expected to be completed by late 2013.
In addition to NIH funding, the Cowlin Family Fund at Chicago Community Trust provided financial support for this study. Zambon donated the NAC and matching placebo; the prednisone, azathioprine, and their matching placebos were purchased using study funds.
Find more information about this clinical trial at http://clinicaltrials.gov/ct2/show/NCT00650091 To arrange an interview with an NHLBI spokesperson, please contact the NHLBI Communications Office at (301) 496-4236 ornhlbi_news@nhlbi.nih.gov.
Part of the National Institutes of Health, the National Heart, Lung, and Blood Institute (NHLBI) plans, conducts, and supports research related to the causes, prevention, diagnosis, and treatment of heart, blood vessel, lung, and blood diseases; and sleep disorders. The Institute also administers national health education campaigns on women and heart disease, healthy weight for children, and other topics. NHLBI press releases and other materials are available online atwww.nhlbi.nih.gov.
21 October, 2011
Prostate Cancer Risk from Vitamin E Supplements
A new study found that vitamin E, once thought to reduce the risk of prostate cancer, may actually increase the risk.

A prostate cancer cell. Image by Anne Weston. All rights reserved by Wellcome Images.
Prostate cancer is the second most common type of cancer in American men. Their current lifetime risk of prostate cancer is 16%. In 2011, there will be an estimated 241,000 new cases of prostate cancer and 34,000 deaths from the disease nationwide
Some early research had suggested that selenium or vitamin E might reduce the risk of developing prostate cancer. To investigate, NIH’s National Cancer Institute (NCI) and several other NIH components funded the Selenium and Vitamin E Cancer Prevention Trial (SELECT). The study began in 2001 and included over 35,000 men.
An initial report in 2008 found that regular intake of vitamin E, vitamin C or selenium does not reduce the risk of prostate cancer or other cancers in older men. The participants were told to stop taking their study supplements and, in 2010, the study sites were closed.
The SELECT researchers had seen a slight increase in prostate cancer risk with vitamin E that may have been due to chance. Over half of the study participants had consented to continue to have their health monitored via mail questionnaires. The researchers’ new analysis included this final data, which was collected through July 2011. Their report appeared on October 12, 2011, in the Journal of the American Medical Association.
The researchers found that men who took 400 international units (I.U.) of vitamin E daily had more prostate cancers than men who took a placebo. For every 1,000 men, there were 76 prostate cancers over a 7-year period among those who took vitamin E supplements vs. 65 in those taking placebo—11 more cases of prostate cancer per 1,000 men. This represents a 17% increase in prostate cancers, a difference not likely due to chance.
The researchers don’t know why vitamin E increased risk instead of decreasing it. SELECT researchers are now measuring the amount of vitamin E, selenium and other nutrients in the blood of participants when they joined the trial to see if the effect of the supplements was affected by baseline levels of these micronutrients. Other researchers are looking at single-letter DNA variations called single nucleotide polymorphisms (SNPs) to see if genetic differences affect a man’s risk of developing prostate cancer while taking vitamin E.
“Based on these results and the results of large cardiovascular studies using vitamin E, there is no reason for men in the general population to take the dose of vitamin E used in SELECT, as the supplements have shown no benefit and some very real risks,” says Dr. Eric Klein, a study co-chair at the Cleveland Clinic. “For now, men who were part of SELECT should continue to see their primary care physician or urologist and bring these results to their attention for further consideration.”
18 October, 2011
Do Baby Products Prevent SIDS? FDA Says No
 The best thing you can do to lower the chance of Sudden Infant Death Syndrome (SIDS) is to place your baby on his or her back to sleep, with nothing else in the crib or bassinet.
The best thing you can do to lower the chance of Sudden Infant Death Syndrome (SIDS) is to place your baby on his or her back to sleep, with nothing else in the crib or bassinet.
That’s the recommendation of the Food and Drug Administration (FDA), which is working to prevent manufacturers of over-the-counter sleep products for babies from claiming that their use will prevent or lower the chance of SIDS. These products include infant positioners, mattresses, crib bedding, pillows, crib tents and baby monitors. Baby products that claim to cure, treat or prevent any condition are considered medical devices, and are subject to FDA regulations designed to protect consumers and patients.
The agency has never approved a product to prevent SIDS—the unexplained death of a baby younger than age 1—and is asking manufacturers to stop marketing their products with these claims until they have received FDA clearance or approval, or to change their labeling to remove all medical claims.
“These products are absolutely not necessary and they can be very dangerous,” says Susan Cummins, M.D., M.P.H., chief pediatric medical officer in FDA’s Center for Devices and Radiological Health.
Dangerous comforts
FDA is aware of 13 infant deaths in the past 13 years associated with sleep positioners, which are used to keep the baby in a desired position. The Consumer Product Safety Commission has received reports of babies found in hazardous positions after being placed in a positioner.
Other products can also be hazardous. Babies can slide down and be trapped by wedges designed to keep them on their back, says Cummins. Blankets, quilts, soft toys and pillow-like crib bedding can smother, she adds.
It’s a matter of A-B-C, says Cummins:
- Alone in their own bed. Don’t keep the infant in your bed next to you and risk that the baby will be accidentally suffocated if you roll over.
- Back to sleep—every sleep. “The safest way to put the baby to sleep is on his or her back every time,” says Cummins. “Do not put the baby on his side or on his stomach.”
Since the national Back to Sleep campaign in 1994 urged parents to place babies on their backs, there has been a 60 percent reduction in SIDS, Cummins says. - Crib. The baby should always be placed in a crib or bassinet to sleep.
Cummins describes the ideal sleep environment for an infant as being free of anything that could block the infant’s movement or breathing. All that’s needed is a firm crib mattress and a tight-fitting sheet.
To parents who have visions of a crib filled with comforts, she says, “Though a crib full of plush toys and soft bedding may look appealing to you, it is hazardous for your baby during his or her first year of life.”
“Your baby will develop faster in that first year than any time after. Newborns can't even hold up their head, yet by their first birthday they are walking or nearly so,” says Cummins. “In between, your baby will learn to roll, sit, turn, crawl and even may start to climb!”
“So in that first year, your baby constantly and rapidly develops new skills, even in the crib during sleep time,” she says. “Make your baby’s crib a safe place to sleep and move, with nothing to get in the way.”
Safe Sleep Resources
FDA is starting a new website on SIDS prevention claims for parents, caregivers and manufacturers of sleep products for babies. Its purpose is to:
- inform parents and caregivers about the risks associated with over-the-counter products that claim to prevent SIDS.
- help manufacturers understand and comply with FDA laws and regulations governing medical devices, which are designed to protect consumers and patients.
The site also offers advice to parents on reducing the risk of SIDS and a list of “baby safe sleep” resources.
“The sleep environment is the one place where the baby is alone, so we want to make sure it’s safe,” says Cummins. And in this case, she says, less is more.
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
17 October, 2011
Organ Donation: Pass it On Give a Gift of Life

A gift with a major impact—one that will long be remembered with gratitude—takes just a bit of preparation. When you become anorgan donor, you can save the lives of up to 8 people. And if you donate tissues like blood cells, bone or corneas, you can help even more.
Organ transplantation was once considered an experimental procedure with a low success rate. Many transplanted organs survived just a few days or weeks. But researchers have transformed transplant surgery from risky to routine. It’s now the treatment of choice for patients with end-stage organ disease. Each day, about 80 Americans receive a lifesaving organ transplant.
“The outcomes of transplantation are really so good these days that it truly makes a difference for the people who receive organ transplants,” says Dr. Sandy Feng, a transplant surgeon at the University of California, San Francisco. “The organs are clearly lifesaving.”
The problem now is that there aren’t enough organs to meet the demand. In early 2011, more than 110,000 people were on the nationwide waiting list for an organ. An average of nearly 20 of them dies each day while waiting.
The kidney is the most commonly transplanted organ. More than 16,000 kidney transplantations were performed in the U.S. last year. The wait, though, can be long. In February 2011, nearly 90,000 people were on the national waiting list for a kidney. Next most commonly transplanted is the liver, with more than 6,000 surgeries in 2010. That’s followed by the heart, lungs, pancreas and intestines.
You can donate some organs—like a kidney or part of your liver—while you’re still alive. You have 2 kidneys but really need only one. And the liver can re-grow if part of it is removed. But donating these organs requires major surgery, which carries risks. That’s why living donors are often family or friends of the transplant recipient.
Most organs, though, are donated after the donor has died. The organs must be recovered quickly after death to be usable. Many come from patients who’ve been hospitalized following an accident or stroke. Once all lifesaving efforts have failed and the patient is declared dead, then organ donation becomes a possibility.
“When a person dies, it can feel like a burden to a family to make decisions about organ donation,” says Feng. “So it would be a real gift to a family to indicate your decision to be an organ donor while you’re still alive, so they don’t have to make the decision for you.”
In addition to organs, you can donate tissues. One of the most commonly transplanted tissues is the cornea, the transparent covering over the eye. A transplanted cornea can restore sight to someone blinded by an accident, infection or disease. Donated skin tissue can be used as grafts for burn victims or for reconstruction after surgery. Donated bones can replace cancerous bones and help prevent amputation of an arm or leg. Donated veins can be used in cardiac bypass surgery.
NIH-funded scientists are exploring a variety of ways to improve organ transplantation. The biggest problem is that when an organ from one person is transplanted to another, the recipient’s immune system attacks the implant as though it’s a disease-causing microbe.
“We’d hit a home run if we could find a way to re-educate a person’s immune system so that it continues to fight infection just as effectively as ever but it didn’t recognize a transplanted organ as foreign. That’s called transplantation tolerance,” says Dr. Nancy Bridges, chief of the transplantation immunology branch at NIH.
To prevent organ rejection, recipients must take drugs, called immunosuppressants, usually for the rest of their lives. “Immunosuppressant drugs have revolutionized our ability to do organ transplantation over the last 30 years,” says Dr. Jerry Nepom, who heads an NIH-funded program called the Immune Tolerance Network. “But those 3 decades have also taught us that these immunosuppressants are not very selective, which is a big problem.”
Immunosuppressants knock down the entire immune system, so that the body has trouble fighting off infections. The drugs also boost the risk for cancer, especially skin cancer. In addition, over time, these potent drugs can damage the kidneys and raise the risk for diabetes, high blood pressure and cardiovascular disease.
“These medications are sort of a necessary evil. You can’t live without them, because you might reject your organ. But it’s difficult to live with them because they cause side effects that need to be managed,” says Feng.
If a patient stops taking immunosuppressants, the transplanted organ nearly always fails. But in very rare cases, people can go off their medications. Last year, NIH-funded scientists spotted a pattern of gene activity in patients who had successfully stopped taking their immunosuppressants after a kidney transplant. Other researchers are testing whether certain liver transplant patients could be weaned off their medications.
“Ultimately, it would be valuable if we could do a blood test to predict who could stop taking their drugs or maybe be on a lower dose,” says Bridges. “We have evidence that it might be possible, but we’re not there yet.”
In other studies, Nepom says, “we’re exploring how to get the recipient’s immune system in a receptive mode, so that it doesn’t become excited and angry when a transplanted organ comes into the body.” In one small clinical study, researchers gave a kidney recipient some of the donor’s bone marrow before surgery. Bone marrow produces cells that fight infection. The procedure created a hybrid immune system in the recipients that better tolerated the transplants. A few patients were able to go off their immunosuppressants within a year after surgery.
While some scientists continue to improve current methods, others are exploring completely new ideas. One cutting-edge approach is to create artificial transplants that won’t trigger an immune system attack. Although years of research will be needed to apply these emerging techniques, researchers have made progress toward engineering livers, lungs and other organs.
You can help reduce the need for donated organs in the first place by living well. Lower your risk of developing a long-term disease that could lead to organ failure by being physically active and eating a healthy diet rich with high-fiber foods, fruits and vegetables. Talk to your doctor about your weight, blood pressure and cholesterol. And while you’re taking these healthy steps, be sure to sign up to be an organ donor so you can help others as well.
Recognizing Schizophrenia Seeking Clues to a Difficult Disorder

What would it be like to hear voices or see people or things that aren’t really there? How would you feel if people seemed out to harm you, and you weren’t sure who to trust? Would you recognize that something was wrong?
Unfortunately, most people with schizophrenia are unaware that their symptoms are warning signs of a mental disorder. Their lives may be unraveling, yet they may believe that their experiences are normal. Or they may feel that they’re blessed or cursed with special insights that others can’t see.
Schizophrenia is a brain disorder that affects about 1 in 100 people. It affects men and women equally in all ethnic groups. Symptoms often start between ages 16 and 30 but most often between 18 and 22. It’s unusual to develop schizophrenia after age 45.
A few decades ago, researchers thought that schizophrenia was caused by inappropriate parenting. Now scientists recognize that a combination of genes and the environment are to blame.
“We know from studies of identical twins that when one twin has schizophrenia, the other twin has a 50% chance of having the disease, indicating that genes may account for half of the mechanisms involved in schizophrenia,” says Dr. José A. Apud, clinical director of the schizophrenia research program at NIH.
But since these twins are genetically the same, other factors must also contribute to schizophrenia. Some scientists have identified environmental factors that may play a role. But researchers don’t yet fully agree on whether or how these factors trigger the disease.
Several genes have been linked to schizophrenia. But each seems to have only a small effect on the chances of getting the disorder. “If we could understand the genes and mechanisms, we might be able to develop drugs that better target the disease,” says Apud.
Although schizophrenia has no cure, 2 main types of treatment can help. “The first line of treatment is always medication, especially antipsychotics,” says Apud. “Second, we use supportive types of psychotherapy and psychosocial treatments.” These can help with everyday living skills and possibly finding an appropriate job.
Patients often try different medications to see which work best. Some types of antipsychotics can cause weight gain, which can lead to diabetes or high cholesterol. Other types can cause a disorder where a person cannot control muscle movements. Despite these drawbacks, antipsychotics greatly improve the lives of most patients.
Problems arise when patients stop taking their medications, which is common. One NIH-funded study found that most patients stop taking antipsychotics within the first 18 months of treatment. “Because of problems with judgment and insight, they may not feel that they need treatment,” Apud says. “Side effects also play a major role in patients’ poor compliance with medications.”
People with schizophrenia often must rely on family or friends to get them into treatment. Caring for and supporting a family member with schizophrenia can be challenging. It may help to find a support group. Talking to others who care for people with schizophrenia may help your whole family.
16 October, 2011
Genes that Influence Blood Pressure
In one of the largest genomic studies ever, an international research consortium identified 29 genetic variations that influence blood pressure. More than half of these variants were previously unknown. The findings provide insights into the biology of blood pressure and may lead to new therapeutic strategies.
High blood pressure, or hypertension, affects over 1 billion people worldwide.

It can damage the body in many ways over time, leading to heart disease, stroke, kidney failure and other health problems.
More than 230 researchers across 6 continents scanned the genomes of over 200,000 European people to identify genetic variants that influence systolic and diastolic blood pressure. They followed up by analyzing the genomes of 70,000 people of East Indian, South Asian and African ancestry. The study was funded by NIH’s National Heart, Lung and Blood Institute (NHLBI), National Institute on Aging (NIA) and National Human Genome Research Institute (NHGRI), among others. The results appeared in the September 11, 2011, issue of Nature.
The researchers discovered 16 previously unknown variations. Six were found in genes already suspected of regulating blood pressure. The remaining 10 were found in unexpected locations and provide new clues into how blood pressure is controlled. Individually, the genetic variations increased the risk of hypertension by only a tiny amount. However, for people with multiple variants, the effects were significant.
The researchers developed a blood pressure genetic risk score based on the 29 variants they found. Among people with the top 10% of genetic risk score, 29% had hypertension, compared with 16% of those in the lowest risk group. Higher genetic risk scores were associated with increased blood pressure across ethnic groups. The risk score was also a good indicator of hypertension complications, such as increased thickness of the heart chambers, heart failure, stroke and coronary artery disease.
“This is one of the most important studies of the genetics of high blood pressure to date and a significant step toward finding better therapies for it,” says NHLBI Acting Director Dr. Susan B. Shurin.
A related study by the research group, the International Consortium of Blood Pressure Genome-Wide Association Studies, appeared on the same day in Nature Genetics. This other genome-wide association study identified 4 new genetic regions associated with pulse pressure and 2 linked to mean arterial pressure. The influence of these variants on systolic and diastolic blood pressure turned out to be more complex than expected.
Taken together, these findings suggest new genetic pathways underlying blood pressure regulation. They will also likely open new doors to research into treating high blood pressure.
Community Immunity
How Vaccines Protect Us All

Parents know that kids are vulnerable to a host of infectious diseases. Research supported by NIH and others proves that the benefits of vaccines in preventing illness and death greatly outweigh the risks.
The list of childhood diseases can be overwhelming: measles, mumps, rubella, diphtheria, pertussis, polio, meningitis, influenza and rotavirus. In the era before vaccines, many children in the U.S. died or became disabled from these diseases. Many still do in countries and regions with lower vaccination rates.
With all the international travel in the world these days, it’s important to keep vaccines, or immunizations, up to date. Here’s just one example of what might happen if you don’t. By 2000, immunization had practically wiped out measles in the U.S. But a measles outbreak in 2005 was traced to one unvaccinated U.S. resident infected during a visit to Europe. The returning traveler infected American children who hadn’t been vaccinated because of safety concerns—despite study after study showing that childhood vaccines are safe and effective.
A major epidemic didn’t emerge that time. That’s because enough people in the surrounding communities had already been vaccinated against measles.
“The important concept,” says Dr. Marc Lipsitch of the Harvard School of Public Health, “is that vaccinating people protects not only them, but others in the community. If I’m protected, I can protect others.”
This type of protection is known as “community immunity” or “herd immunity.” When enough of the community is immunized against a contagious disease, most other members are protected from infection because there’s little opportunity for the disease to spread.
Newborns, pregnant women or people whose immune systems are weakened may not be eligible for certain vaccines. Yet even they will get some protection because the spread of contagious disease is contained.
“Epidemiologists think of infections as chain reactions, whose speed depends on contagiousness,” says Lipsitch. “The more contagious the disease, the more vaccination is required. The data tells us that herd immunity works.”
Using mathematical formulas and computer programs, NIH-funded scientists like Lipsitch have developed models to determine what proportion of the population has to be vaccinated to eliminate the spread of disease. As one example, a worldwide vaccination campaign completely eliminated, or eradicated, smallpox in the 1970s. So many people were immunized that the virus couldn’t sustain itself.
More recently, infant vaccination against Haemophilus influenzae type b (Hib, which can cause meningitis) lowered the risk of disease in the whole population. Before the vaccine, Hib struck about 1 in 200 children younger than age 5. It killed many and often left survivors with permanent brain damage. After the Hib vaccine was introduced in the mid-1980s, the incidence of Hib dropped by 99%.
“Infectious disease eradication is possible,” says Lipsitch. Even when a disease—such as measles or Hib— hasn’t been completely wiped out, immunizations can reduce disease transmission, so that epidemics become less frequent.
When parents choose to immunize, they’re helping more than their own. Make sure your child’s immunizations are up to date. And talk with your child’s doctor if you have any concerns about vaccine safety.
Inefficient developing world stoves contribute to 2 million deaths a year
International effort could reduce death toll, deforestation, NIH scientists say
An international effort to replace smoky, inefficient household stoves that people commonly use in lower and middle income countries with clean, affordable, fuel efficient stoves could save nearly 2 million lives each year, according to experts from the National Institutes of Health.
In a commentary in Science, the NIH scientists noted that indoor air pollution from such inefficient stoves affects about 3 billion people—nearly half the world's population. In addition to respiratory disease caused by smoke, the fuel needed by inefficient stoves leads to—deforestation, and environmental degradation.
"Many people in developed countries don't realize that smoke from indoor cooking fires is a terrible scourge upon the health of a large number of people," said Francis Collins, M.D., Ph.D, director of the National Institutes of Health and an author of the study. "International efforts to combat this scourge are now beginning. The NIH's role is to support the research that will determine the most efficient, cost effective means to do so while safe guarding human health."
The study authors stated that nearly half the world's population uses biomass (wood, crop residues, charcoal or dung) or coal as fuel for cooking and heating. "The primitive fires typically fill homes with dense smoke, blackening walls and ceilings and sickening those within."
Other authors of the study were William J. Martin II, M.D., associate director for prevention research and health promotion at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Roger I. Glass, M.D., Ph.D., director of the Fogarty International Center, and John M. Balbus, senior advisor for public health, National Institute of Environmental Health Sciences.
Women and children are at greatest risk for the adverse health effects posed by inefficient stoves, the study authors wrote. Men tend to leave home during the day, but women and children remain. As a result, women and children have many of the same disease risks as do people who smoke tobacco. These risks include pneumonia, lung cancer and chronic obstructive pulmonary disease.
In many societies, women and girls typically gather fuel for the stoves. Fuel gathering is time-consuming and, because they must often walk several miles from the safety of their home communities, these women and girls are at increased risk for gender-based violence.
"By freeing up time, efficient stoves can even expand the opportunities for education and economic development of women and girls in these impoverished areas," the scientists wrote.
The study authors cited a recent report by the World Bank, which noted that, in addition to improving public health, clean, efficient stoves could have benefits to the environment and the climate, by reducing carbon dioxide emissions.
In recognition of the problem, the United Nations launched the Global Alliance for Clean Cookstoves. A public-private partnership, the alliance seeks to create a global market for clean and efficient cookstoves and fuels in the developing world. The alliance’s target is "100 by 20," which stands for the adoption of clean, efficient stoves and fuels by 100 million homes by the year 2020, with eventual worldwide adoption. The authors noted that the U.S. government has committed more than $50 million to the effort, including about $25 million in research funds for the NIH.
To succeed, strategies for replacing the world's inefficient biomass stoves with clean, efficient stoves must be market driven, the researchers added. So that cleaner stoves will be accepted, they must meet the needs of those who will use them.
"Promoting sustained changes in the way food is cooked to reduce [indoor air pollution] requires a fundamental understanding of traditions, social interactions, and family dynamics, which differ widely across cultures," the authors wrote. "Successful implementation invariably involves women in stove design, training, use in the home, and follow-up in the community."
Local manufacture of new stoves would have the added benefit of stimulating local economic development.
Educating people about the health risks of the stoves would also increase demand, as potential users understand that the initial expense of a more efficient stove would have health benefits in the long run, the authors wrote. Governmental subsidies to help the poorest people purchase the stoves would provide additional incentive, as would efforts to informal local peoples that the new stoves would cut household fuel costs.
The authors also called for more research on the potential health benefits of cleaner, more efficient stoves. It is not precisely known how much emissions must be reduced to produce health benefits. For example, preliminary data from one study suggest that reducing exposure to emissions by 90 percent is needed to substantially reduce the risk of pneumonia, and reducing exposure by 50 percent is required to modestly reduce the risk. Similarly, such studies could confirm the link between indoor air pollution and suspected health risks such as low birth weight, cataracts, cardiovascular disease, asthma, and tuberculosis.
The authors noted, however, that programs to replace inefficient stoves are already under way and that these programs have been undertaken without advance research into their potential benefits. Along with research to determine the amount of reduction in indoor air pollution needed to be effective, programs are also needed to evaluate the potential benefits of programs now under way.
The authors estimated the costs of a research program to on health and indoor air pollution to range from $150 million to $200 million. Although these costs might seem high, the authors wrote, they are typical of the costs of research investments needed to combat leading worldwide causes of death.
"The challenges are great, but the potential to use a relatively low cost intervention to save millions of lives, improve the environment, and encourage economic development is compelling," the researchers concluded.
Fogarty, the international component of the NIH, addresses global health challenges through innovative and collaborative research and training programs and supports and advances the NIH mission through international partnerships. For more information, visit: www.fic.nih.gov.
The National Institute of Environmental Health Sciences supports research to understand the effects of the environment on human health and is part of NIH. For more information on environmental health topics, visit our Web site at http://www.niehs.nih.gov.
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): The NICHD sponsors research on development, before and after birth; maternal, child, and family health; reproductive biology and population issues; and medical rehabilitation. For more information, visit the Institute's Web site athttp://www.nichd.nih.gov/.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visitwww.nih.gov.
14 October, 2011
13 October, 2011
Gene Variant Affects Response to Asthma Drugs
A genetic variant may explain why some people with asthma don’t respond well to inhaled corticosteroids, the most widely prescribed medicine for long-term asthma control. In the future, knowledge of such variants could help doctors develop more effective, personalized asthma treatments.

Asthma is a complex inflammatory disease that affects more than 22 million people nationwide. It causes narrowing of the airways in the lungs and leads to wheezing, coughing, chest tightness and trouble breathing. Many factors can influence how severely the disease affects people and how well they respond to treatments.
A poor response to inhaled corticosteroids often runs in families, so scientists have suspected that genetics plays a role. To learn more, a team of researchers led by Dr. Kelan G. Tantisira of Brigham and Women's Hospital carried out a genome-wide association study of children with asthma and their parents. The scientists searched for genetic variants linked to a poor response to inhaled corticosteroids. The study was funded by NIH’s National Heart, Lung and Blood Institute (NHLBI), National Human Genome Research Institute (NHGRI) and the NIH Pharmacogenomics Research Network, among others.
The investigators first ran a genome-wide scan of the DNA of 118 children with asthma and their parents. As reported in the September 26, 2011, online edition of the New England Journal of Medicine, the researchers uncovered a variant in the gene called glucocorticoid-induced transcript 1 (GLCCI1) that appeared to be associated with a poor response to inhaled corticosteroids.
The scientists verified this association in 935 additional children and adults with asthma who had enrolled in 4 independent studies. About 1 in 6 study participants had 2 copies of the GLCCI1 variant. Compared to those with 2 copies of the regular GLCCI1 gene, patients carrying 2 copies of the variant were more than twice as likely to respond poorly to inhaled corticosteroids. Those with a poor response had an average of one-third the level of lung improvement from inhaler treatment as those with 2 regular copies of the gene.
In laboratory cultures, the scientists saw changes caused by the GLCCI1variant that might explain why it leads to a decreased response to inhaled corticosteroids. However, more studies will be needed to better understand how the variant operates in the lungs.
The researchers estimate that the GLCCI1 variant accounts for about 6.6% of the overall variation to inhaled corticosteroids between people. Other factors that are yet to be discovered doubtless influence a person’s response to these drugs. Also, because most of the study participants were white, the results may not be applicable to people of other ethnicities. More studies will be needed to explore whether GLCCI1 contributes to corticosteroid response in other ethnic groups.
“This finding helps to explain the genetic basis for the long-standing observation that some people do not respond well to what is a common asthma treatment,” says NHLBI Acting Director Dr. Susan Shurin. “The study illustrates the importance of research examining the relationship between genetic makeup and response to therapy for asthma, and underscores the need for personalized treatment for those who have it.”
Ovarian Cancer Treatment: Where We Are Now
AUGUST 2, 2011, 3:46PM
By Aleea Farrakh Khan
Ovarian cancer has proven to be a very difficult cancer to diagnose at a curable stage and thus treat successfully. Even though it has one of the highest mortality rates of all gynecological cancers in the United States, there are no validated or proven screening tests, making it a challenge to diagnose at an early stage. To date, there is no evidence that any of the various screening tests that are performed, including pelvic examinations, transvaginal ultrasounds and a CA-125 assay (a test that measures the level of CA-125 in the blood to see if it is elevated), leads to a decrease in ovarian cancer deaths. These tests have not been shown to diagnose ovarian cancer early, and the risk of falsely calling a benign mass a cancer when it is not present is unacceptably high. This can lead to unnecessary surgery, treatments, and stress for patients.
Ovarian cancer symptoms are fairly non-specific, therefore only about 19 percent of all cases are detected at an early, localized stage. In the U.S. alone, an estimated 22,000 women will be diagnosed with, and 15,000 women will die from this disease in 2011. Even with all these challenges, researchers have made important clinical advances over the years in chemotherapy regimens, surgery techniques and biologic therapies to find better treatment options for ovarian cancer patients.
Three-panel drawing of stage IA, IB, and IC ovarian cancer. Credit: Terese Winslow
The image above depicts stage IA, IB, and IC ovarian cancer. The first panel shows a stage IA tumor inside one ovary. The second panel shows two stage IB tumors, one inside each ovary. The third panel shows two stage IC tumors, one inside each ovary, and one tumor has a ruptured capsule. An inset shows cancer cells floating in the peritoneal fluid surrounding abdominal organs.
The primary surgical objective in ovarian cancer treatment is removal of the tumor. It has been shown consistently that the more complete the resection, or removal of the tumor, the better the clinical outcome since the current surgical aim is removal of disease to the point that there is no visible disease present. However, not every woman can undergo such surgery. This fact led to a trial, recently reported by the European Organization for Research and Treatment of Cancer–Gynecologic Cancer Group (EORTC–GCG) and the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) examining the question of whether surgery should precede chemotherapy or if chemotherapy should come first, a method called neoadjuvant chemotherapy. Results demonstrated that there was no difference between the approaches and that neoadjuvant chemotherapy could be considered, given the similar survival outcomes and the increased side-effects of primary surgery. Newer GOG trials will allow physicians and their patients to elect neoadjuvant therapy or traditional chemotherapy after primary surgical debulking, or removal of the malignancies.
Cisplatin crystals, a platinum compound used as a chemotherapy drug.
In 1978, the U.S. FDA approved cisplatin, a chemotherapy drug containing platinum, for treatment of metastatic ovarian cancer. Shortly after that approval, the delivery of anticancer drugs intraperitoneally (IP) was established—a technique where chemotherapy drugs are administered through a surgically implanted catheter, allowing passage of fluids into the abdominal cavity. This method allows direct administration of drugs to the intra-abdominal cancer, creating higher local drug exposure. Some of the drugs used intraperitoneally, such as cisplatin and its close relative carboplatin, are also absorbed into the general circulation and assist with attacking ovarian cancer that has spread to other parts of the body.
The next significant pharmaceutical advancement was the approval of paclitaxel in the mid-90’s; the first of a class of drugs known as taxanes. Paclitaxel interferes with cell growth and division in rapidly dividing cells, such as cancer cells. Unlike the platinums, the taxanes do not get absorbed when administered into the abdominal cavity and thus provide high local drug exposure.
Researchers studied both cisplatin and paclitaxel extensively and found that chemotherapy regimens that contain both types of drugs are most effective in preventing recurrence of ovarian cancer and improving a woman’s survival period. The combination of cisplatin and paclitaxel has become the standard recommended therapy for treatment for women with ovarian cancer who may benefit from chemotherapy. Further improving this method in 2006, a study by the Gynecologic Oncology Group (GOG) showed that women with advanced ovarian cancer who receive a combination of intravenous (IV) and IP chemotherapy post-surgery extended their overall survival by about a year. The combined method of delivering drugs into the vein and directly into the abdomen simultaneously allows for improved progression-free survival. It is possible that this was due to the intra-abdominal administration of the drugs; however, more total chemotherapy was administered on the combination IV and IP therapy arm, suggesting that quantity of drug, known as dose density, may be the factor.
Biologic therapy is another area of interest that is being explored for ovarian cancer treatments. Researchers continue to study the benefits of targeted agents in the form ofmonoclonal antibodies and small molecules to treat a number of other cancers, including ovarian cancer. Anti-angiogenic agents, a form of targeted therapy that uses small molecule drugs or antibodies to stop tumors from making new blood vessels, have also shown promise in clinical settings. Trials are currently underway to investigate whether the addition of bevacizumab, a type of anti-angiogenic drug, to first-line treatment will improve clinical outcomes. In 2010, a study by the Gynecologic Oncology Group (GOG) found that women who received bevacizumab (Avastin) during their initial chemotherapy for ovarian cancer and continued up to 16 months after completion of initial chemotherapy had a reduced risk of progression of 28 percent compared to those who received chemotherapy alone. The benefit was short-lived leaving the community in a quandary regarding application of this therapy as a new standard of care. A newer study, reported this spring, the OCEANS trial, added bevacizumab to carboplatin and gemcitabine for women with first recurrence of ovarian cancer. There was a greater reduction in risk of progression in this study and an improvement in overall survival. How these studies will change practice patterns for the future is not yet known.
One of the most exciting recent advances in ovarian cancer has been the discovery and use of a new class of targeted agents, the PARP inhibitors. Olaparib, one type of PARP inhibitor, blocks the activity of PARP1 and PARP2 proteins that are necessary for cells to repair damaged DNA. This agent was found to be clinically active in breast and ovarian cancer patients who carry germ line mutations in the BRCA1 or 2 genes, and olaparib has also been active in high grade serous ovarian cancer, a type of epithelial ovarian cancer. Researchers hope that combining a PARP inhibitor, like olaparib, with traditional chemotherapy drugs, such as the platinums, will produce greater anticancer effects than either chemotherapy or a PARP inhibitor alone. This approach is based on the observation that cells are unable to survive if they accumulate high levels of DNA damage. Additional PARP-inhibitors are now under development and their roles are being investigated in women who are both BRCA1 and 2 mutation carriers and other women with ovarian cancer.
Acknowledging the poor prognosis of ovarian cancer, The Cancer Genome Atlas (TCGA) sponsored by the NCI, selected serous ovarian cancer, the most prevalent form of the disease, as one of the first to have its genomic changes charted in depth. The goal of the TCGA profiling was to look for gene expression patterns that are linked to differences in patient survival and to establish whether certain gene changes can be linked to response to therapy. To date, TCGA has achieved comprehensive sequencing, characterization, and analysis of the genomic changes in ovarian cancer. Their initial findings, just reported in the journal Nature, were of interest to many investigators. It showed that there are no frequent driving genetic mutations in ovarian cancer as has been shown in many other solid tumors. Serous ovarian cancer distinguished itself by its genetic complexity and variability. Investigators are now combing this remarkable data collection to identify leads for typing ovarian cancer in ways that will focus therapy for greater clinical benefit, survival advantages, and to reduce toxicity and patient injury.
11 October, 2011
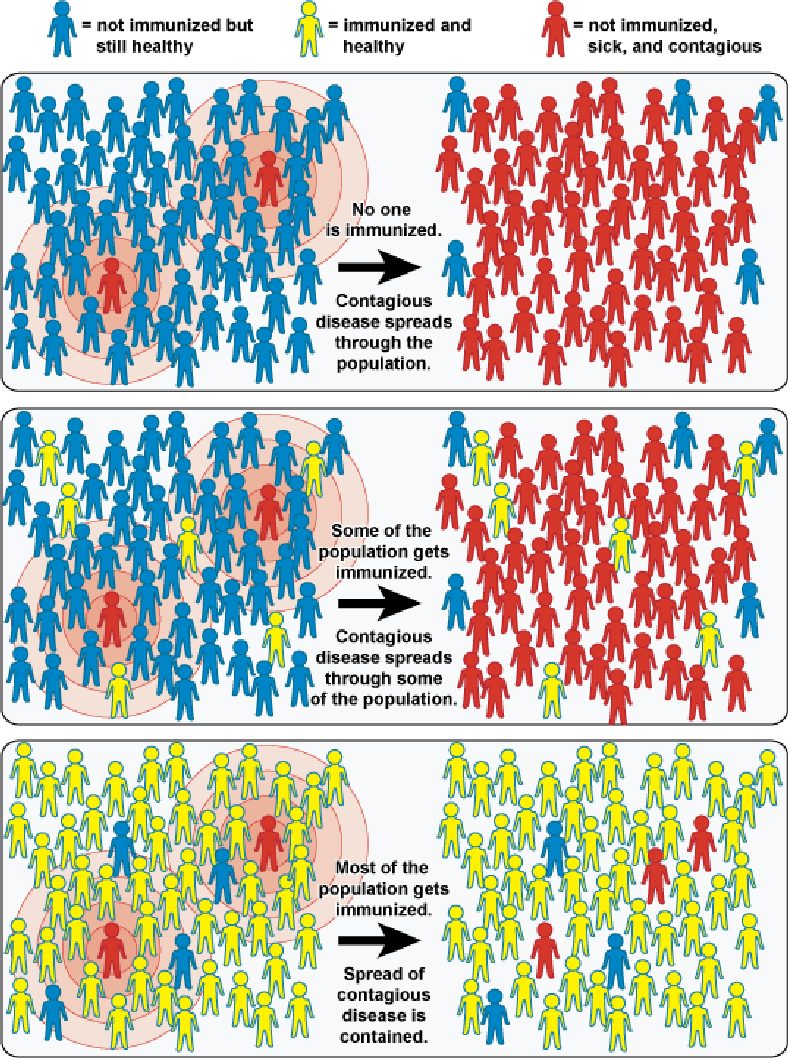
Community Immunity ("Herd" Immunity)
Vaccines can prevent outbreaks of disease and save lives.
When a critical portion of a community is immunized against a contagious disease, most members of the community are protected against that disease because there is little opportunity for an outbreak. Even those who are not eligible for certain vaccines—such as infants, pregnant women, or immunocompromised individuals—get some protection because the spread of contagious disease is contained. This is known as "community immunity."
In the illustration below, the top box depicts a community in which no one is immunized and an outbreak occurs. In the middle box, some of the population is immunized but not enough to confer community immunity. In the bottom box, a critical portion of the population is immunized, protecting most community members.
The principle of community immunity applies to control of a variety of contagious diseases, including influenza, measles, mumps, rotavirus, and pneumococcal disease.
10 October, 2011
Unite in the fight against NCDs - September 2011
07 October, 2011
Genomic tools prove integral to solving medical mysteries
IH Undiagnosed Diseases Program documents two-year pilot as clinic of last resort
Louise Benge, Brodhead, Ky., a participant in the NIH Undiagnosed Diseases Program, is monitored while walking. Annette Stine, research coordinator, National Heart Lung and Blood Institute, NIH, monitors the treadmill test.

Bethesda, Md., Thurs., Oct. 6, 2011 — After its first two years of work, the Undiagnosed Diseases Program (UDP) of the National Institutes of Health is citing successes in patients whose cases have stumped specialists at leading medical institutions around the country. The researchers published the program's first retrospective analysis in the Sept. 26, 2011 early online issue of Genetics in Medicine.
The successes include the diagnoses of siblings whose calcium-riddled blood vessels made it excruciatingly painful to walk, a woman with life-threatening protein deposits in her muscles and a 20-year-old whose diagnosis makes him the oldest survivor of his previously undiagnosed muscle and lung disorder.
"The UDP responds to a critical unmet need, with compassion, clinical expertise and state of the art genomic technologies," said Daniel Kastner, M.D., Ph.D. , scientific director at the National Human Genome Research Institute (NHGRI). "A patient who cannot be diagnosed may cycle through the medical system with no satisfactory treatment plan or be abandoned by the medical system. Through the UDP, NIH provides a glimmer of hope to patients and their families, while at the same time gaining remarkable medical insights."
The UDP is supported by the NIH Office of the Director, NHGRI, the NIH Office of Rare Diseases Research (ORDR) and the NIH Clinical Center.
The report focuses on 160 patients of the total 326 cases accepted into the program. More than half of the accepted patients had undiagnosed neurological problems. Other prominent disorder categories include gastrointestinal disease; fibromyalgia and chronic fatigue syndrome; immune-mediated and rheumatic illnesses; psychiatric conditions; pain; dermatologic disorders; and cardiovascular disease.
So far, most of the solved cases — 37 of 39 cases for which the UDP team arrived at a diagnosis — involved diseases previously encountered in the world of medicine, according to UDP authors. In general, about 500 diseases are common enough to be in any physician's repertoire for diagnosis, while another 6,500 are known but are exceptionally rare, according to ORDR data.
UDP researchers reviewed, evaluated and diagnosed 23 patients with rare diseases, of which 15 cases reflect extremely rare diseases affecting fewer than 10,000 people. The authors note that while these are known disorders, some lack diagnostic tests or medical definitions to describe them. Rare diseases are defined as those affecting fewer than 200,000 people in the United States.
The program has also delved into the realm of unknown maladies. In February, the UDP announced the program's first discovery of a new disease, called ACDC, or arterial calcification due to deficiency of CD73, in the New England Journal of Medicine. CD73 is a protein that produces a small molecule, adenosine, which protects arteries from calcifying. A report on one additional new disorder is pending publication.
The siblings whose cases led to discovery of ACDC continue to experience pain while walking more than a short distance. The NIH researchers, however, have obtained approval to start a drug treatment protocol that could improve their condition, which will be initiated within months.
The patient who UDP researchers encountered with an unexplained muscle condition was diagnosed with a rare form of amyloidosis, a condition in which bone marrow produces excess immunoglobulin proteins, which had accumulated in the patient's muscle tissue. The NIH team referred the patient for a stem-cell, bone marrow transplant, using healthy donor stem cells. The patient has subsequently experienced progressive improvement in her condition.
The UDP team also succeeded in diagnosing the 20-year-old patient with a condition called spinal muscular atrophy with respiratory distress. The condition causes damage to muscles, including respiratory muscles. The patient remains dependent on a respirator for much of his day but last year achieved the significant personal milestone of high school graduation. The diagnosis has allayed the patient's concern that the condition might at any point impair his learning.
UDP's novel approach
A typical UDP patient visits the NIH Clinical Center for one week. The case is evaluated by specialists from several of NIH's 27 institutes and centers, with expertise in areas such as neurology, radiology, dentistry and rheumatology. A key component of the program is genetics, so researchers collect DNA from blood or tissue samples from all participating patients, and often from family members to support the genomic analyses.
Most of the patients accepted in the first two years of the UDP had their DNA analyzed for known single nucleotide polymorphisms (SNPs), which reflect differences in the single chemical subunits of DNA that could indicate a genetic disorder. Their tool in the SNP analysis process is called a million-SNP array, which can be used to find potentially important differences between the genome of an affected individual and an unaffected family member, pointing to the genetic cause of a disorder. This approach resulted in three successful diagnoses.
The researchers performed both whole-genome sequencing, deciphering all of an individual's DNA code, and whole-exome sequencing, an approach that decodes the 1-2 percent of the genome that contains protein-coding genes. They analyzed DNA from 32 patients, along with DNA from 78 unaffected family members. This approach proved critical for the diagnoses of six patients' disorders.
NIH is evaluating use of these advanced genomic analyses for broader utility. The UDP diagnostic successes have proven the usefulness of SNP detection techniques and genome sequencing tools — both whole genome and whole exome sequencing — in the clinical evaluation of patients, according to the study. But UDP researchers also know that genome sequencing does not provide the whole answer. In addition to genomic analyses, clinical findings — from specialty consults to radiological tests — led to one third of the 39 diagnoses, according to the study.
A flood of applications
Doctors from around the country responded to the May 2008 call for UDP applications, summarizing, documenting and sending the UDP 1,191 cases for review within the subsequent two years alone. Each application includes a referring health care provider summary letter and complete medical records.
"The applications may represent years of evaluation by multiple doctors at more than one medical facility — but with no conclusive diagnosis," said William Gahl, M.D., Ph.D., NHGRI clinical director and UDP director. "We look for some clue in the medical record — from an abnormal lab test to a collection of symptoms that don't usually occur together. If we can establish a direction for further follow up, we may invite that patient to be seen by our team at NIH."
NIH clinicians participating in UDP — up to 60 health care providers at the nation's largest research hospital — screen the applications and accept patients based on the availability of clinical and research resources. The program currently has a backlog of applications and since July 2011 has suspended acceptance of new applications until November 2011.
"In addition to our discovery of new disorders, the UDP work has expanded the clinical description — or phenotype — of numerous disorders," Dr. Gahl said. "The limited rate of diagnosis during the program is sobering. While we wish we could arrive at a conclusive diagnosis for each patient, the reality is that many of their conditions are likely new diseases and we continue to pursue clues long after patients depart the hospital here at NIH." To increase the success rates, the UDP plans to make case descriptions available to designated expert researchers, to both validate findings and enhance understanding of disorders.
NHGRI is one of the 27 institutes and centers at the NIH, an agency of the Department of Health and Human Services. The NHGRI Division of Intramural Research develops and implements technology to understand, diagnose and treat genomic and genetic diseases. Additional information about NHGRI can be found at its website, www.genome.gov.
05 October, 2011
ANTI ANGIOGENESIS: A NEW FRONTIER IN CANCER THERAPY, BETTER HOPE FOR THE PEOPLE WITH CANCER.
Authors: Tisa Cherian, Bijina Hameed, Teenu Anne Tony, Pushpagiri College of Pharmacy, Thiruvalla. INDIA
ABSTRACT:
Cancer cells are having the ability to colonize and invade new areas in the body. In order to grow and metastasis, a tumor cell must have adequate blood supply. They achieve this by stimulating angiogenesis which supply nutrition to the tumor. Tumor angiogenesis is a complicated process that begins when the cancer cells release substances called growth factors such as VEGF (vascular endothelial growth factor). Anti-angiogenesis is based on the shutdown of ability of tumor cells to form new blood vessels, thereby cutting its source of food. One of the foremost benefits of targeted therapies is reduced toxicity and improved quality of life. Several anti-angiogenic drugs are undergoing the clinical trials for the treatment of cancer. Anti-angiogenesis therapy has proven to be more effective when compared to radiational therapy as radiational therapy results in damage of subsidiary cells which in turn lead to prevalence on necrosis.
INTRODUCTION:
Cancer is a large, heterogeneous class of diseases in which a group of cells display uncontrolled growth, invasion that intrudes upon and destroys adjacent tissues, and often metastasize, wherein tumor cells spread to other locations in the body through the lymphatic system and or through the blood stream. Angiogenesis is a natural process through which the body develops new blood vessels, and is thought to play a central role in the growth factors, VEGF (vascular endothelial growth factor).After the release of VEGF it attaches to nearby cells which triggers new blood vessels to sprout towards the tumor. These blood vessels provide the tumor with a steady blood supply and nutrients. By means of anti-angiogenic therapy, it provides the action by inhibiting the supply of blood vessels to the tumor prone areas.
ANGIOGENESIS AND CANCER:
Angiogenesis is a physiological process involved in the growth of new blood vessels from pre-existing vessels. Different forms include, sprouting and intussusceptive angiogenesis.
By creating their own network of blood vessels, tumors develop an independent and reliable source of nutrients and oxygen which feeds the tumor.
To spread they need to be supplied by blood vessels that bring oxygen and nutrients and remove metabolic wastes.
ANGIOGENIC SEQUENCE:
The angiogenic process takes place by the following sequences;
A cell activated by lack of oxygen release angiogenic molecules that attract inflammatory and endothelial cells and promote their proliferation.
During their migration, inflammatory cells also secrete molecules that intensify the angiogenic stimuli.
The endothelial cells that form the blood vessels respond to the angiogenic call by differentiating and by secreting matrix metalloproteases (MMP), which digest the blood-vessel walls to enable them to escape and migrate toward the site of the angiogenic stimuli.
Several protein fragments produced by the digestion of the blood-vessel walls intensify the proliferative and migratory activity of endothelial cells, which then form a capillary tube by altering the arrangement of their adherence-membrane proteins.
Finally, through the process of anastomosis, the capillaries emanating from the arterioles and the venules will join, thus resulting in a continuous blood flow.
ANTI ANGIOGENESIS THERAPY:
Anti-angiogenic treatment is designed to prevent the growth of new blood vessels. Without these blood vessels, the tumor no longer has a way to get the oxygen and nutrients needed to survive and grow. The history has evolved in 1970 Dr. Judah Folkman introduced the idea of anti-angiogenic agent as possible tool in the fight against cancer. In 1980 researches were able to identify VEGF growth factor which starts the growth of blood vessels; this in turn leads to the discovery of new anti-angiogenic agents.
Ø Anti-angiogenic drugs work by stopping the growth of new blood vessels – starving tumors of the blood and nutrients essential for growth.
Ø Inhibitors may be natural or synthetic include protese inhibitors (tissue inhibitors of matrix metalloproteinases), tyrosine kinase inhibitors, interleukins and proteolytic fragment of diverse molecules (endostatin, vasostatin, arrestin,..)
Ø Anti-angiogenic drugs prevent the vascular endothelial growth factor from binding with the receptors on the surface of the endothelial cells.
Ø Act by impeding the growth of endothelial cells inside the tumor, which in turn prevent the proliferation of tumor. So these target the blood vessels which in turn impede the development of epithelial cells in tumor.
Ø Also act in multiple ways, inhibition of endothelial cell proliferation, migration, by protease activity, tubule formation and induction of apoptosis.
Ø Angiogenic inhibitors can act synergistically with conventional treatments and tends to have non overlapping toxicities.
Ø Different anti-angiogenic drugs act by different mechanism.
Ø
| INHIBITORS | USES | MECHANISMS |
| Bevacizumab | Cancer | Binds VEGF |
| VEGFR antagonist | Prostate cancer | Inhibit binding of angiogenic stimulators |
| Matrix metalloproteinase inhibitors | | Inhibit basement membrane degradation |
| Tetrathiomolybdate | Cancer | Copper chelation which inhibit blood vessel growth |
Ø Avastin is the first Anti-angiogenic drug developed for cancer therapy. It’s an antibody that targets the VEGF that is released by growing tumor cells, inhibiting it. Avastin in combination with intravenous 5-flurouracil-based chemotherapy is indicated for first or second line treatment of patients with metastatic carcinoma of the colon and rectum.
Ø Human diets can also act as mild angiogenesis inhibitors such as soy products, (which contain the inhibitor genistein), black raspberry extract, green tea (catechins), liquorice (glycyrrhizic acid), red wine (resveratrol).
MECHANISM OF ACTION:
Anti-angiogenic agent binds directly to the VEGF ligand to prevent its interaction with receptors on the surface of endothelial cells thereby inhibiting the biological activity of VEGF.
CONCLUSION:
Although the use of anti-angiogenesis drugs in cancer treatment strategy is still in its infancy, they have a great potential. The anti-angiogenesis approach bears fewer side effects and is generally a less risky mode of treatment. Anti-angiogenesis drugs pose no risk of a chemo brain and subsequent brain damage or of the Alzheimer’s disease for some genetically predisposed patients. As research goes on, we can today hope with greater trust that our loved ones will be saved from the throes of cancerous pain.
BIBLIOGRAPHY:
· Journal of the National Cancer Institute (JNCI).
· Journal of Young Investigators.
· American Journal of Human Genetics.
· www.newfrontierin cancer.org
COURTESY: Tisa Cherian