14 October, 2011
13 October, 2011
Gene Variant Affects Response to Asthma Drugs
A genetic variant may explain why some people with asthma don’t respond well to inhaled corticosteroids, the most widely prescribed medicine for long-term asthma control. In the future, knowledge of such variants could help doctors develop more effective, personalized asthma treatments.
Asthma is a complex inflammatory disease that affects more than 22 million people nationwide. It causes narrowing of the airways in the lungs and leads to wheezing, coughing, chest tightness and trouble breathing. Many factors can influence how severely the disease affects people and how well they respond to treatments.
A poor response to inhaled corticosteroids often runs in families, so scientists have suspected that genetics plays a role. To learn more, a team of researchers led by Dr. Kelan G. Tantisira of Brigham and Women's Hospital carried out a genome-wide association study of children with asthma and their parents. The scientists searched for genetic variants linked to a poor response to inhaled corticosteroids. The study was funded by NIH’s National Heart, Lung and Blood Institute (NHLBI), National Human Genome Research Institute (NHGRI) and the NIH Pharmacogenomics Research Network, among others.
The investigators first ran a genome-wide scan of the DNA of 118 children with asthma and their parents. As reported in the September 26, 2011, online edition of the New England Journal of Medicine, the researchers uncovered a variant in the gene called glucocorticoid-induced transcript 1 (GLCCI1) that appeared to be associated with a poor response to inhaled corticosteroids.
The scientists verified this association in 935 additional children and adults with asthma who had enrolled in 4 independent studies. About 1 in 6 study participants had 2 copies of the GLCCI1 variant. Compared to those with 2 copies of the regular GLCCI1 gene, patients carrying 2 copies of the variant were more than twice as likely to respond poorly to inhaled corticosteroids. Those with a poor response had an average of one-third the level of lung improvement from inhaler treatment as those with 2 regular copies of the gene.
In laboratory cultures, the scientists saw changes caused by the GLCCI1variant that might explain why it leads to a decreased response to inhaled corticosteroids. However, more studies will be needed to better understand how the variant operates in the lungs.
The researchers estimate that the GLCCI1 variant accounts for about 6.6% of the overall variation to inhaled corticosteroids between people. Other factors that are yet to be discovered doubtless influence a person’s response to these drugs. Also, because most of the study participants were white, the results may not be applicable to people of other ethnicities. More studies will be needed to explore whether GLCCI1 contributes to corticosteroid response in other ethnic groups.
“This finding helps to explain the genetic basis for the long-standing observation that some people do not respond well to what is a common asthma treatment,” says NHLBI Acting Director Dr. Susan Shurin. “The study illustrates the importance of research examining the relationship between genetic makeup and response to therapy for asthma, and underscores the need for personalized treatment for those who have it.”
Ovarian Cancer Treatment: Where We Are Now
AUGUST 2, 2011, 3:46PM
By Aleea Farrakh Khan
Ovarian cancer has proven to be a very difficult cancer to diagnose at a curable stage and thus treat successfully. Even though it has one of the highest mortality rates of all gynecological cancers in the United States, there are no validated or proven screening tests, making it a challenge to diagnose at an early stage. To date, there is no evidence that any of the various screening tests that are performed, including pelvic examinations, transvaginal ultrasounds and a CA-125 assay (a test that measures the level of CA-125 in the blood to see if it is elevated), leads to a decrease in ovarian cancer deaths. These tests have not been shown to diagnose ovarian cancer early, and the risk of falsely calling a benign mass a cancer when it is not present is unacceptably high. This can lead to unnecessary surgery, treatments, and stress for patients.
Ovarian cancer symptoms are fairly non-specific, therefore only about 19 percent of all cases are detected at an early, localized stage. In the U.S. alone, an estimated 22,000 women will be diagnosed with, and 15,000 women will die from this disease in 2011. Even with all these challenges, researchers have made important clinical advances over the years in chemotherapy regimens, surgery techniques and biologic therapies to find better treatment options for ovarian cancer patients.
Three-panel drawing of stage IA, IB, and IC ovarian cancer. Credit: Terese Winslow
The image above depicts stage IA, IB, and IC ovarian cancer. The first panel shows a stage IA tumor inside one ovary. The second panel shows two stage IB tumors, one inside each ovary. The third panel shows two stage IC tumors, one inside each ovary, and one tumor has a ruptured capsule. An inset shows cancer cells floating in the peritoneal fluid surrounding abdominal organs.
The primary surgical objective in ovarian cancer treatment is removal of the tumor. It has been shown consistently that the more complete the resection, or removal of the tumor, the better the clinical outcome since the current surgical aim is removal of disease to the point that there is no visible disease present. However, not every woman can undergo such surgery. This fact led to a trial, recently reported by the European Organization for Research and Treatment of Cancer–Gynecologic Cancer Group (EORTC–GCG) and the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) examining the question of whether surgery should precede chemotherapy or if chemotherapy should come first, a method called neoadjuvant chemotherapy. Results demonstrated that there was no difference between the approaches and that neoadjuvant chemotherapy could be considered, given the similar survival outcomes and the increased side-effects of primary surgery. Newer GOG trials will allow physicians and their patients to elect neoadjuvant therapy or traditional chemotherapy after primary surgical debulking, or removal of the malignancies.
Cisplatin crystals, a platinum compound used as a chemotherapy drug.
In 1978, the U.S. FDA approved cisplatin, a chemotherapy drug containing platinum, for treatment of metastatic ovarian cancer. Shortly after that approval, the delivery of anticancer drugs intraperitoneally (IP) was established—a technique where chemotherapy drugs are administered through a surgically implanted catheter, allowing passage of fluids into the abdominal cavity. This method allows direct administration of drugs to the intra-abdominal cancer, creating higher local drug exposure. Some of the drugs used intraperitoneally, such as cisplatin and its close relative carboplatin, are also absorbed into the general circulation and assist with attacking ovarian cancer that has spread to other parts of the body.
The next significant pharmaceutical advancement was the approval of paclitaxel in the mid-90’s; the first of a class of drugs known as taxanes. Paclitaxel interferes with cell growth and division in rapidly dividing cells, such as cancer cells. Unlike the platinums, the taxanes do not get absorbed when administered into the abdominal cavity and thus provide high local drug exposure.
Researchers studied both cisplatin and paclitaxel extensively and found that chemotherapy regimens that contain both types of drugs are most effective in preventing recurrence of ovarian cancer and improving a woman’s survival period. The combination of cisplatin and paclitaxel has become the standard recommended therapy for treatment for women with ovarian cancer who may benefit from chemotherapy. Further improving this method in 2006, a study by the Gynecologic Oncology Group (GOG) showed that women with advanced ovarian cancer who receive a combination of intravenous (IV) and IP chemotherapy post-surgery extended their overall survival by about a year. The combined method of delivering drugs into the vein and directly into the abdomen simultaneously allows for improved progression-free survival. It is possible that this was due to the intra-abdominal administration of the drugs; however, more total chemotherapy was administered on the combination IV and IP therapy arm, suggesting that quantity of drug, known as dose density, may be the factor.
Biologic therapy is another area of interest that is being explored for ovarian cancer treatments. Researchers continue to study the benefits of targeted agents in the form ofmonoclonal antibodies and small molecules to treat a number of other cancers, including ovarian cancer. Anti-angiogenic agents, a form of targeted therapy that uses small molecule drugs or antibodies to stop tumors from making new blood vessels, have also shown promise in clinical settings. Trials are currently underway to investigate whether the addition of bevacizumab, a type of anti-angiogenic drug, to first-line treatment will improve clinical outcomes. In 2010, a study by the Gynecologic Oncology Group (GOG) found that women who received bevacizumab (Avastin) during their initial chemotherapy for ovarian cancer and continued up to 16 months after completion of initial chemotherapy had a reduced risk of progression of 28 percent compared to those who received chemotherapy alone. The benefit was short-lived leaving the community in a quandary regarding application of this therapy as a new standard of care. A newer study, reported this spring, the OCEANS trial, added bevacizumab to carboplatin and gemcitabine for women with first recurrence of ovarian cancer. There was a greater reduction in risk of progression in this study and an improvement in overall survival. How these studies will change practice patterns for the future is not yet known.
One of the most exciting recent advances in ovarian cancer has been the discovery and use of a new class of targeted agents, the PARP inhibitors. Olaparib, one type of PARP inhibitor, blocks the activity of PARP1 and PARP2 proteins that are necessary for cells to repair damaged DNA. This agent was found to be clinically active in breast and ovarian cancer patients who carry germ line mutations in the BRCA1 or 2 genes, and olaparib has also been active in high grade serous ovarian cancer, a type of epithelial ovarian cancer. Researchers hope that combining a PARP inhibitor, like olaparib, with traditional chemotherapy drugs, such as the platinums, will produce greater anticancer effects than either chemotherapy or a PARP inhibitor alone. This approach is based on the observation that cells are unable to survive if they accumulate high levels of DNA damage. Additional PARP-inhibitors are now under development and their roles are being investigated in women who are both BRCA1 and 2 mutation carriers and other women with ovarian cancer.
Acknowledging the poor prognosis of ovarian cancer, The Cancer Genome Atlas (TCGA) sponsored by the NCI, selected serous ovarian cancer, the most prevalent form of the disease, as one of the first to have its genomic changes charted in depth. The goal of the TCGA profiling was to look for gene expression patterns that are linked to differences in patient survival and to establish whether certain gene changes can be linked to response to therapy. To date, TCGA has achieved comprehensive sequencing, characterization, and analysis of the genomic changes in ovarian cancer. Their initial findings, just reported in the journal Nature, were of interest to many investigators. It showed that there are no frequent driving genetic mutations in ovarian cancer as has been shown in many other solid tumors. Serous ovarian cancer distinguished itself by its genetic complexity and variability. Investigators are now combing this remarkable data collection to identify leads for typing ovarian cancer in ways that will focus therapy for greater clinical benefit, survival advantages, and to reduce toxicity and patient injury.
11 October, 2011
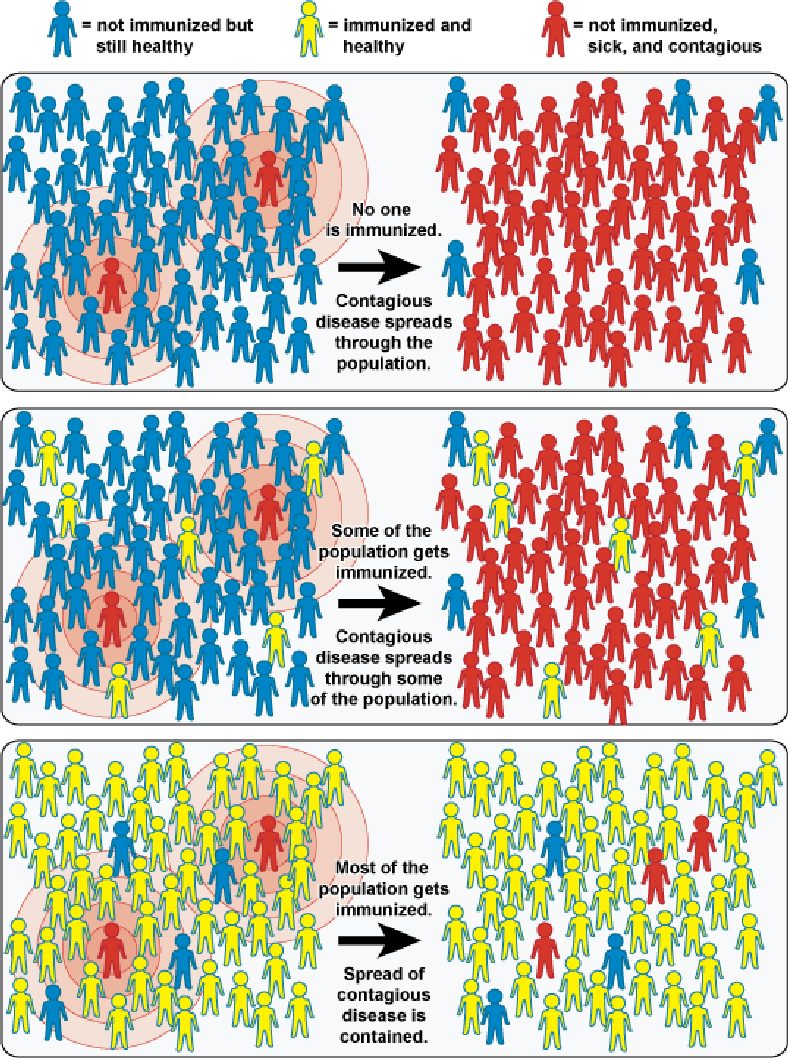
Community Immunity ("Herd" Immunity)
Vaccines can prevent outbreaks of disease and save lives.
When a critical portion of a community is immunized against a contagious disease, most members of the community are protected against that disease because there is little opportunity for an outbreak. Even those who are not eligible for certain vaccines—such as infants, pregnant women, or immunocompromised individuals—get some protection because the spread of contagious disease is contained. This is known as "community immunity."
In the illustration below, the top box depicts a community in which no one is immunized and an outbreak occurs. In the middle box, some of the population is immunized but not enough to confer community immunity. In the bottom box, a critical portion of the population is immunized, protecting most community members.
The principle of community immunity applies to control of a variety of contagious diseases, including influenza, measles, mumps, rotavirus, and pneumococcal disease.
10 October, 2011
Unite in the fight against NCDs - September 2011
07 October, 2011
Genomic tools prove integral to solving medical mysteries
IH Undiagnosed Diseases Program documents two-year pilot as clinic of last resort
Louise Benge, Brodhead, Ky., a participant in the NIH Undiagnosed Diseases Program, is monitored while walking. Annette Stine, research coordinator, National Heart Lung and Blood Institute, NIH, monitors the treadmill test.
Bethesda, Md., Thurs., Oct. 6, 2011 — After its first two years of work, the Undiagnosed Diseases Program (UDP) of the National Institutes of Health is citing successes in patients whose cases have stumped specialists at leading medical institutions around the country. The researchers published the program's first retrospective analysis in the Sept. 26, 2011 early online issue of Genetics in Medicine.
The successes include the diagnoses of siblings whose calcium-riddled blood vessels made it excruciatingly painful to walk, a woman with life-threatening protein deposits in her muscles and a 20-year-old whose diagnosis makes him the oldest survivor of his previously undiagnosed muscle and lung disorder.
"The UDP responds to a critical unmet need, with compassion, clinical expertise and state of the art genomic technologies," said Daniel Kastner, M.D., Ph.D. , scientific director at the National Human Genome Research Institute (NHGRI). "A patient who cannot be diagnosed may cycle through the medical system with no satisfactory treatment plan or be abandoned by the medical system. Through the UDP, NIH provides a glimmer of hope to patients and their families, while at the same time gaining remarkable medical insights."
The UDP is supported by the NIH Office of the Director, NHGRI, the NIH Office of Rare Diseases Research (ORDR) and the NIH Clinical Center.
The report focuses on 160 patients of the total 326 cases accepted into the program. More than half of the accepted patients had undiagnosed neurological problems. Other prominent disorder categories include gastrointestinal disease; fibromyalgia and chronic fatigue syndrome; immune-mediated and rheumatic illnesses; psychiatric conditions; pain; dermatologic disorders; and cardiovascular disease.
So far, most of the solved cases — 37 of 39 cases for which the UDP team arrived at a diagnosis — involved diseases previously encountered in the world of medicine, according to UDP authors. In general, about 500 diseases are common enough to be in any physician's repertoire for diagnosis, while another 6,500 are known but are exceptionally rare, according to ORDR data.
UDP researchers reviewed, evaluated and diagnosed 23 patients with rare diseases, of which 15 cases reflect extremely rare diseases affecting fewer than 10,000 people. The authors note that while these are known disorders, some lack diagnostic tests or medical definitions to describe them. Rare diseases are defined as those affecting fewer than 200,000 people in the United States.
The program has also delved into the realm of unknown maladies. In February, the UDP announced the program's first discovery of a new disease, called ACDC, or arterial calcification due to deficiency of CD73, in the New England Journal of Medicine. CD73 is a protein that produces a small molecule, adenosine, which protects arteries from calcifying. A report on one additional new disorder is pending publication.
The siblings whose cases led to discovery of ACDC continue to experience pain while walking more than a short distance. The NIH researchers, however, have obtained approval to start a drug treatment protocol that could improve their condition, which will be initiated within months.
The patient who UDP researchers encountered with an unexplained muscle condition was diagnosed with a rare form of amyloidosis, a condition in which bone marrow produces excess immunoglobulin proteins, which had accumulated in the patient's muscle tissue. The NIH team referred the patient for a stem-cell, bone marrow transplant, using healthy donor stem cells. The patient has subsequently experienced progressive improvement in her condition.
The UDP team also succeeded in diagnosing the 20-year-old patient with a condition called spinal muscular atrophy with respiratory distress. The condition causes damage to muscles, including respiratory muscles. The patient remains dependent on a respirator for much of his day but last year achieved the significant personal milestone of high school graduation. The diagnosis has allayed the patient's concern that the condition might at any point impair his learning.
UDP's novel approach
A typical UDP patient visits the NIH Clinical Center for one week. The case is evaluated by specialists from several of NIH's 27 institutes and centers, with expertise in areas such as neurology, radiology, dentistry and rheumatology. A key component of the program is genetics, so researchers collect DNA from blood or tissue samples from all participating patients, and often from family members to support the genomic analyses.
Most of the patients accepted in the first two years of the UDP had their DNA analyzed for known single nucleotide polymorphisms (SNPs), which reflect differences in the single chemical subunits of DNA that could indicate a genetic disorder. Their tool in the SNP analysis process is called a million-SNP array, which can be used to find potentially important differences between the genome of an affected individual and an unaffected family member, pointing to the genetic cause of a disorder. This approach resulted in three successful diagnoses.
The researchers performed both whole-genome sequencing, deciphering all of an individual's DNA code, and whole-exome sequencing, an approach that decodes the 1-2 percent of the genome that contains protein-coding genes. They analyzed DNA from 32 patients, along with DNA from 78 unaffected family members. This approach proved critical for the diagnoses of six patients' disorders.
NIH is evaluating use of these advanced genomic analyses for broader utility. The UDP diagnostic successes have proven the usefulness of SNP detection techniques and genome sequencing tools — both whole genome and whole exome sequencing — in the clinical evaluation of patients, according to the study. But UDP researchers also know that genome sequencing does not provide the whole answer. In addition to genomic analyses, clinical findings — from specialty consults to radiological tests — led to one third of the 39 diagnoses, according to the study.
A flood of applications
Doctors from around the country responded to the May 2008 call for UDP applications, summarizing, documenting and sending the UDP 1,191 cases for review within the subsequent two years alone. Each application includes a referring health care provider summary letter and complete medical records.
"The applications may represent years of evaluation by multiple doctors at more than one medical facility — but with no conclusive diagnosis," said William Gahl, M.D., Ph.D., NHGRI clinical director and UDP director. "We look for some clue in the medical record — from an abnormal lab test to a collection of symptoms that don't usually occur together. If we can establish a direction for further follow up, we may invite that patient to be seen by our team at NIH."
NIH clinicians participating in UDP — up to 60 health care providers at the nation's largest research hospital — screen the applications and accept patients based on the availability of clinical and research resources. The program currently has a backlog of applications and since July 2011 has suspended acceptance of new applications until November 2011.
"In addition to our discovery of new disorders, the UDP work has expanded the clinical description — or phenotype — of numerous disorders," Dr. Gahl said. "The limited rate of diagnosis during the program is sobering. While we wish we could arrive at a conclusive diagnosis for each patient, the reality is that many of their conditions are likely new diseases and we continue to pursue clues long after patients depart the hospital here at NIH." To increase the success rates, the UDP plans to make case descriptions available to designated expert researchers, to both validate findings and enhance understanding of disorders.
NHGRI is one of the 27 institutes and centers at the NIH, an agency of the Department of Health and Human Services. The NHGRI Division of Intramural Research develops and implements technology to understand, diagnose and treat genomic and genetic diseases. Additional information about NHGRI can be found at its website, www.genome.gov.